
Phase Diagram of Water: In this typical phase diagram of water, the green lines mark the freezing point, and the blue line marks the boiling point, showing how they vary with pressure. The critical temperature for oxygen is -118✬, so oxygen cannot be liquefied above this temperature. The curve ends at a point called the critical point, because at higher temperatures the liquid phase does not exist at any pressure.

A pressure cooker (or even a covered pot) will cook food faster because the water can exist as a liquid at temperatures greater than 100º C without all boiling away. As the pressure increases, the boiling temperature rises steadily to 374º C at a pressure of 218 atm. For example, the boiling point of water is 100º C at 1.00 atm.

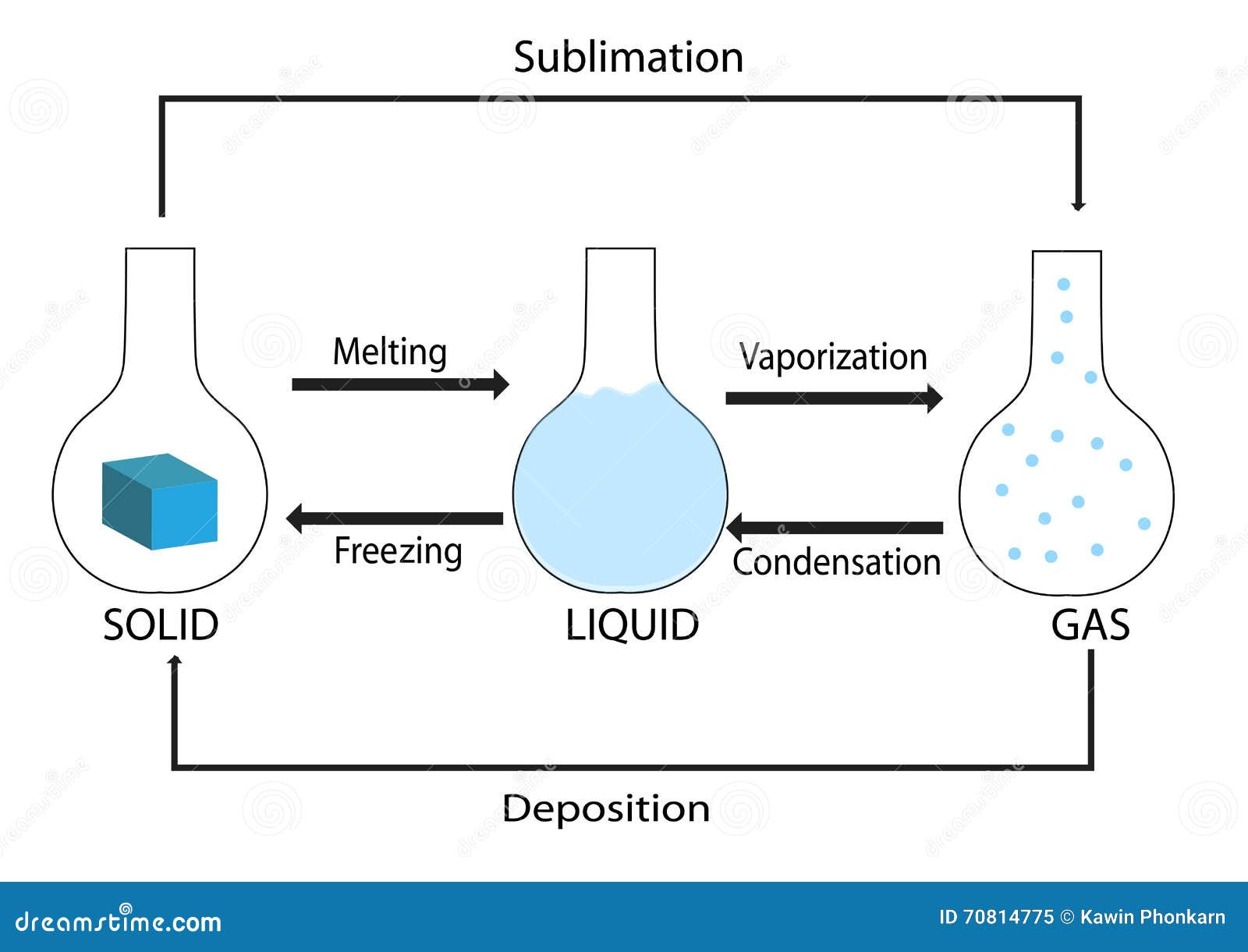
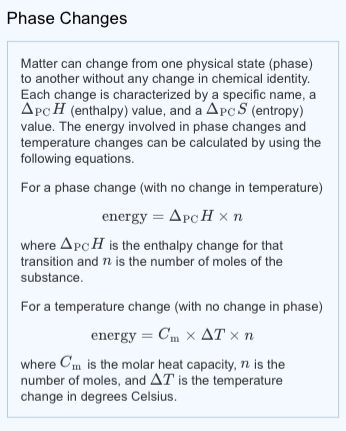
The solid lines-boundaries between phases-indicate temperatures and pressures at which the phases coexist (that is, they exist together in ratios, depending on pressure and temperature). Using the graph, if you know the pressure and temperature you can determine the phase of water. There are well-defined regions on these graphs that correspond to various phases of matter, so PT graphs are called phase diagrams. The plots of pressure versus temperatures provide considerable insight into thermal properties of substances. Heat: This graph shows the temperature of ice as heat is added. Once all water has been boiled to steam, the temperature will continue to rise linearly as heat is added. At the boiling point, temperature no longer rises with heat added because the energy is once again being used to break intermolecular bonds. Once all ice has been melted, the temperature again rises linearly with heat added. At this point, there is a mixture of both ice and water. But the heat added does not change the temperature that heat energy is instead used to break intermolecular bonds and convert ice into water. Temperature increases linearly with heat, until the melting point. If that amount of energy is added to a mole of that substance at boiling or freezing point, all of it will melt or boil, but the temperature won’t change. These amounts of energy are the molar heat of vaporization and molar heat of fusion. To boil or melt one mole of a substance, a certain amount of energy is required. This is because once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy. Only after it has completely evaporated will it get any hotter. The term is most commonly used to describe transitions between solid, liquid and gaseous states of matter and, in rare cases, plasma.Īs an example, if you boil water, it never goes above 100 degrees Celsius. The measurement of the external conditions at which the transformation occurs is termed the phase transition. For example, a liquid may become gas upon heating to the boiling point, resulting in an abrupt change in volume. During a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external condition, such as temperature or pressure. Describe behavior of the medium during a phase transitionĪ phase of a thermodynamic system and the states of matter have uniform physical properties.


 0 kommentar(er)
0 kommentar(er)
